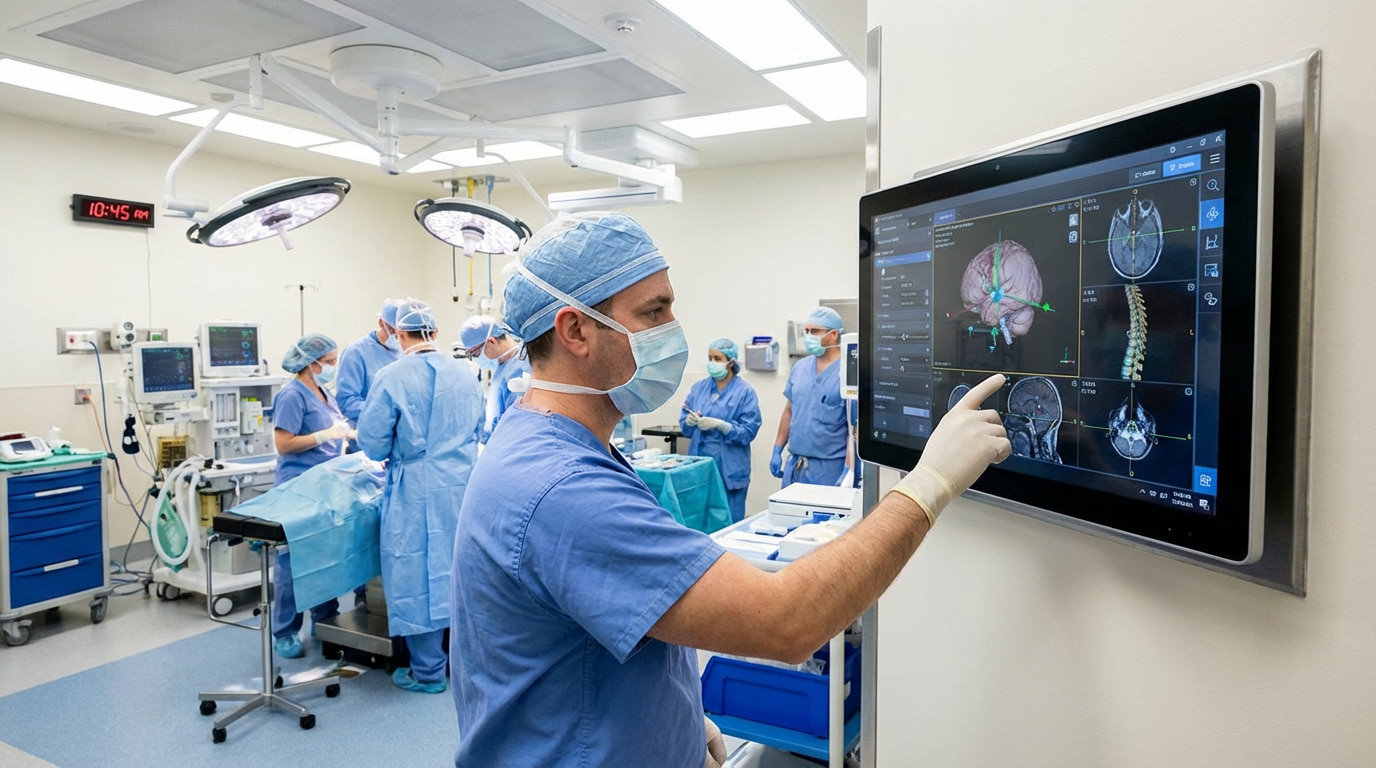
The core highlight of Higgstec’s certification lies in the inclusion of Design within the scope. This means we integrate stringent medical device design and manufacturing control processes right from the project initiation phase.
This capability allows us to collaborate closely with clients during the early stages of product development, providing highly integrated and customized medical touchscreen solutions. This approach significantly mitigates potential risks and complexities for our clients during subsequent product validation and regulatory submission processes.
Our Quality Management System (QMS) certificate was issued by IMQ (Istituto Italiano del Marchio di Qualità – Italian Quality Mark Institute) and is recognized by the IQNET International Certification Network. This certification, which carries high international credibility, provides robust regulatory support for our clients targeting global markets, particularly in Europe.
Furthermore, Higgstec’s operational footprint includes a production base in Su’ao, Yilan, and a technical support center in Guishan, Taoyuan. This multi-site integrated operation model, combined with rigorous supplier management mechanisms, ensures stability and flexibility in both capacity allocation and supply chain management. This structure is essential for meeting the stringent demands of the medical industry regarding quality and delivery timelines.
Built upon a foundation of strict risk management, Higgstec focuses intensely on process validation and software verification and validation (V&V). The development and manufacturing processes for our touchscreen solutions are specifically designed to help clients' end products comply with international medical safety regulations. We position ourselves not merely as a component supplier, but as a trusted technical partner throughout our clients' medical device development lifecycle.
➡︎[IMQ] ISO 13485 - Overview
➡︎[Click Here to Download Certificate]